



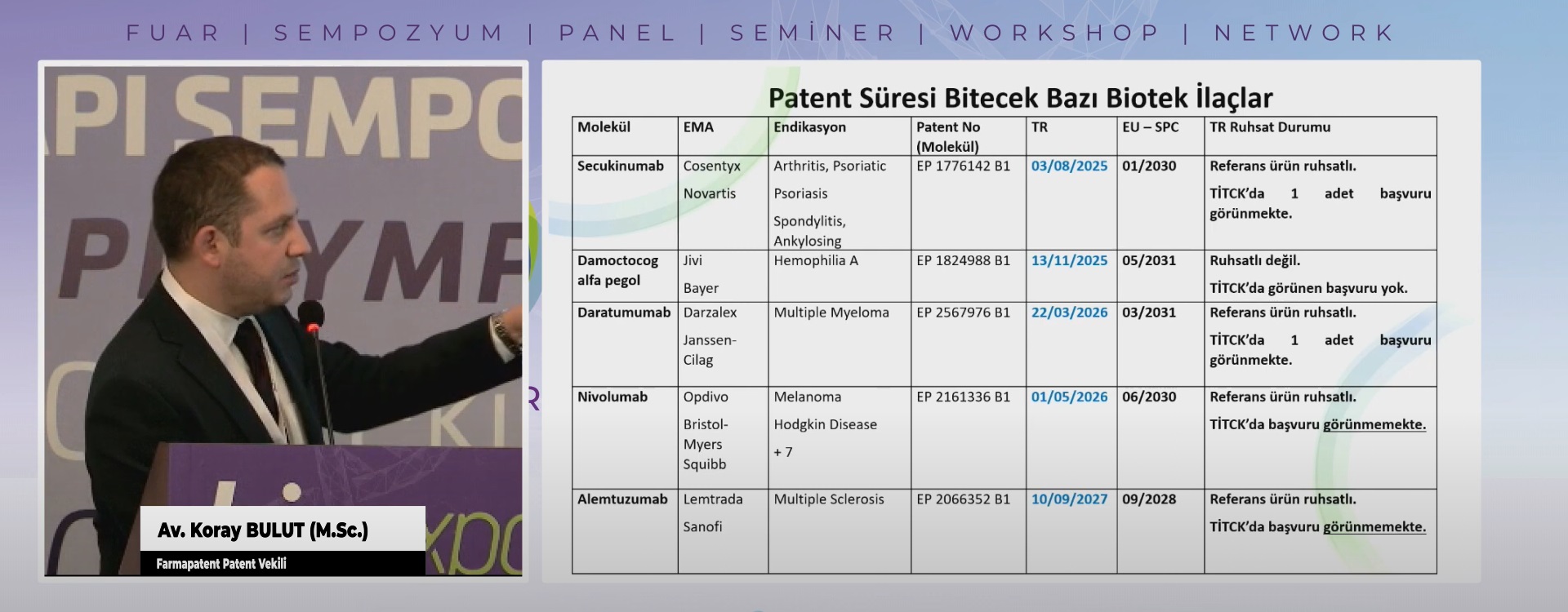

Patent Araştırma Raporları Patent araştırma raporlarının amacı; geliştirme aşamasında ola...
Detaylar
Faaliyet Serbestisi Çalışmaları Faaliyet Serbestisi Çalışmaları bir ürünün, üçüncü kişile...
Detaylar
Patent Tecavüz Analizleri Tecavüz analizleri, bir ürünün/prosesin üçüncü kişiye ait bir p...
Detaylar
Patent Hükümsüzlük Analizleri Hükümsüzlük analizleri, bir patentin hükümsüz kılınma ihtim...
Detaylar
Patentlenebilirlik Araştırmaları Patent başvurusundan önce, buluşun patent olarak tescil ...
Detaylar